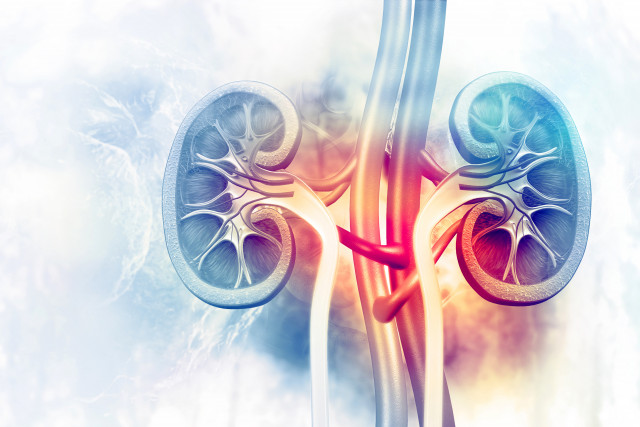
ENYO Pharma Announces a €39 Million Series C Financing and FDA Clearance to Advance Vonafexor in a Phase 2 Clinical Trial for Patients With Alport Syndrome
ENYO Pharma (“ENYO”) announced that it has received clearance of its Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) to initiate a Phase 2 clinical…


![[✨NEW✨#걸어서세계속으로📺] 각양각색의 매력을 간직한 '세계의 정원 여행' (KBS_20231230)](https://i1.ytimg.com/vi/ldcXF85q-0w/hqdefault.jpg)