 |
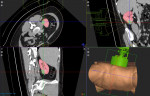
HistoSonics, Inc., (www.histosonics.com), developer of a non-invasive platform and novel sonic beam therapy called histotripsy, announced today the treatment of the first patient in its pivotal #HOPE4KIDNEY Trial. The FDA approved the investigational device study earlier last year and it is designed to evaluate the safety and efficacy of the company’s breakthrough Edison System to destroy targeted kidney tumors, completely non-invasively and without the need for needles or incisions. The #HOPE…